A business’s primary goal in most cases is to make profits. That being said, sustainable profits can only occur with expansion, which is why many ventures explore new markets to capture additional market share. The above statement applies to all sectors, and the pharmaceutical industry is no exception.
For pharma companies, venturing into new markets, especially emerging markets, presents a unique opportunity to make good returns for a multitude of reasons.
In this post, we explore the intricacies of venturing into new markets for pharma companies and the role of regulators in all of this.
Photo by Michael Longmire on Unsplash
Steps involved when venturing into new markets
Market entry could either be local or international, so the plan of action involved in each case could differ to some extent. But irrespective of the geographical region any business seeks to enter, specific fundamentals that start from — market research to developing a market entry strategy, and finally executing said entry strategy — needs to be followed.
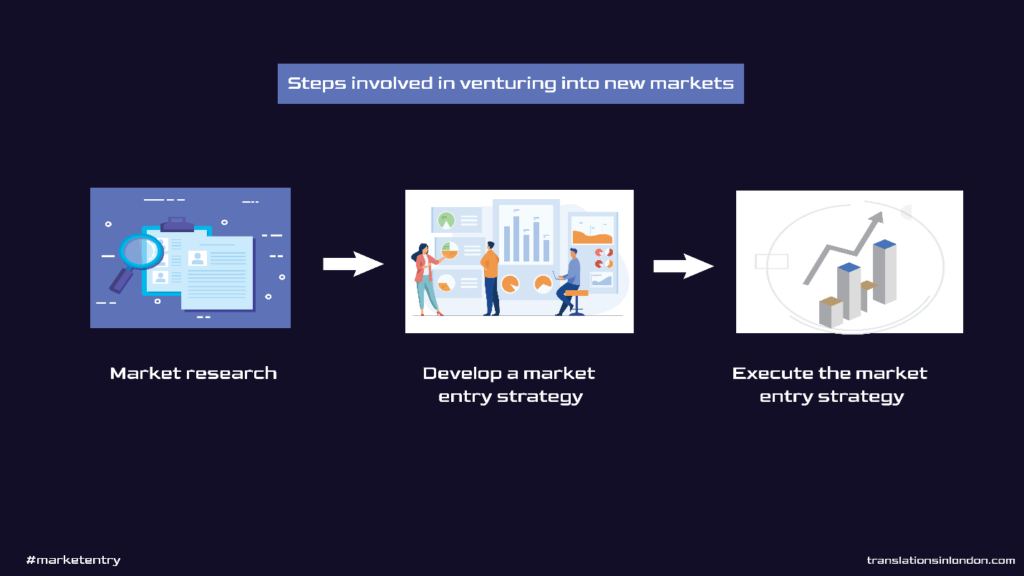
Steps involved in market entry
Barriers pharma companies face during market entry
Import regulations, government protectionism, high costs, complex distribution network, etc., are SOME of the barriers to market entry.
One major obstacle at play, especially for pharmaceutical companies, would be the role of the drug regulator in the country of entry. While some emerging markets may not have strict drug regulation policies, developed markets like the US and EU have regulatory bodies (FDA and EMA) that impose strict guidelines on pharmaceutical companies before they can be allowed to access these markets.
These guidelines are put in place to ensure the high safety and efficacy standards of all produced drugs.
Role of pharmaceutical regulations
The need for safe drugs that are of the highest quality and effectiveness has justified the need for pharma regulations.
There is also the need to reduce asymmetric information between pharma companies and regulatory bodies on the quality of drugs entering the market to a minimum in order to forestall harm due to improper administration of said drugs to patients.
Some may argue that this could be the bane of the strict policy requirement that serves as a barrier for market entry on the part of pharma companies.
The effect of some of these strict policies come in various forms. In a previous post, we touched on the low number of drug approvals in the U.S., but polices could also be in the form of the high cost of carrying out research and development for new drugs. After all, it is said that the average cost of R&D to get a successful new drug into the market stands around $2.8 Billion.
The primary purpose of regulators like the FDA and EMA is the role of granting drug approval to pharma companies. The process of doing so involves a lot of information moving back and forth between both parties involved. In cases involving a foreign company, pharmaceutical translation services are mandatory in overcoming language barriers to ensure that no information is lost during translation.
The process of granting regulatory approval to pharma companies would involve the regulator to carry out activities like;
- Quality Control
Regulatory bodies are, in most cases, required by law to monitor the quality of drugs pharma companies are attempting to bring into their local market. For instance, the FDA has the Current Good Manufacturing Practice (CGMP) regulation to maintain minimum requirements in methods, facilities, and controls employed in the manufacture of specific drugs. CGMP compliance is a requirement for regulatory approval by the FDA.
- Another role pharma regulation plays in the drug approval process is the review and evaluation of drug data. It involves activities like the review of;
- Drug test results
- Data from animal and human clinical trials
- Drug effectiveness compared to information submitted by pharma companies
- Manufacturer information to ensure manufacturing compliance
What does it all mean?
By now, it must be clear to all that the process of getting drug approval from pharma regulators is not a stroll in the park. The time consuming and cost-intensive nature of the entire process warrants that every step must be precise with no error whatsoever.
This implies that pharma companies, asides from adhering to all regulatory requirements, must ensure that no information is lost along the way by properly navigating language barriers in healthcare to be able to tap into the rewards of both emerging and developed markets.
Do you have plans to venture into new markets both in the local and international scene?
Translationsinlondon has a long list of professionally certified translators ready to help you take your business into any of the foreign markets.
Get in touch with us today by sending us an email

